Back
It is not unique to have a little confusion with all the CDC, CMS and IDOH changes related to COVID-19 guidance and requirements that just keep coming. Below is a reminder of the last CMS revision related to the requirements of COVID-19 testing for nursing homes.
- Facilities have two options to conduct outbreak testing, through either a contact tracing or broad-based testing approach. See Table 1.
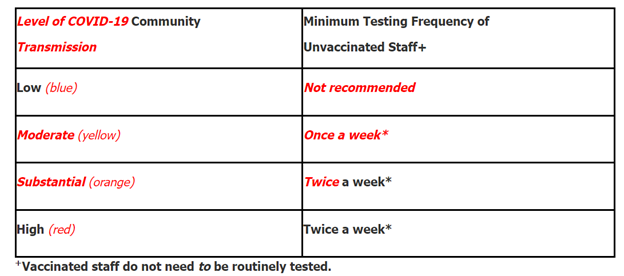
- Routine staff testing is based on the facility’s county level of community transmission. The frequency of testing has also been updated. See Table 2.
Table 1:
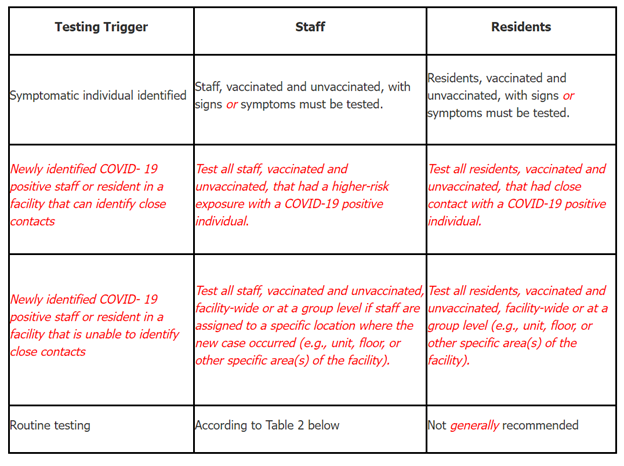
Table 2:
IHCA encourages nursing home providers to read the entire QSO memorandum for complete details:
For questions, please contact Lori Davenport at ldavenport@ihca.org
About the Author
Lori Davenport, Director of Clinical & Regulatory Affairs


