On August 26, 2020, the Centers for Medicare and Medicaid Services (CMS) issued QSO-20-38, providing guidance on the Interim Final Rule released on August 25, 2020, and its requirements for COVID-19 testing of nursing home staff and residents. For IHCA/INCAL’s original summary on the Interim Final Rule, please click here. QSO 20-38 addresses the requirements for when nursing facilities are required to test staff and residents as a requirement of participation in Medicare and Medicaid and provides a revised survey tool for surveyors to assess compliance with the new testing requirements. While the Interim Final Rule was released on August 25, 2020, it is not final until published in the Federal Register. It is expected to be published on Wednesday, September 2, 2020 and at that time the regulation and guidance becomes effective.
Nursing facilities may comply with the testing requirement through the use of rapid point-of-care (POC) diagnostic testing devices or through an arrangement with an offsite laboratory, although IHCA/INCAL is still waiting for further guidance from the Indiana State Department of Health (ISDH) regarding the reporting of POC test results. Only antigen tests and PCR tests are permitted to be used to meet the testing requirement – antibody tests are not permitted.
COVID-19 Testing of Nursing Facility Staff & Residents
QSO 20-38 requires testing based on three (3) different triggers:
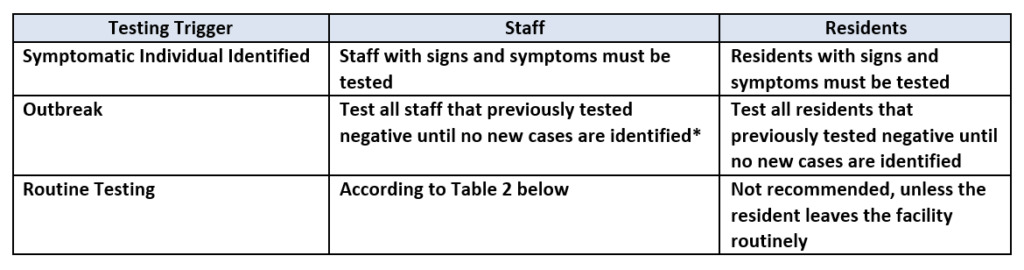
- Symptomatic Individual Identified: Staff with signs or symptoms should be restricted from the facility pending the results of the test, and if the test is positive, the staff member should adhere to the CDC’s Return to Work Criteria. Residents with signs or symptoms should be placed on Transmission-Based Precautions until the results are returned and appropriate action can be taken based on the results.
- Outbreak: When any new case arises in a facility among staff or there is a nursing home-onset case in a resident, all staff and residents should be tested, and all staff and residents that tested negative should be tested every three (3) to seven (7) days until no new cases are identified among staff or residents for a period of at least 14 days since the most recent positive result. The term “nursing home-onset” refers to a case of COVID-19 that originated in a nursing facility and does not refer to the following:
- Residents who were known to have COVID-19 on admission to the facility and were placed into appropriate Transmission-Based Precautions.
- Residents who were placed into Transmission-Based Precautions on admission and developed COVID-19 within 14 days of admission.
- Routine Testing: Routine testing must be conducted of staff according to the table below based on the facility’s county positivity rate in the prior week. As noted above, county-level positivity rates will be available at the following website starting August 28, 2020. Routine testing of residents is not recommended unless a resident leaves the facility routinely.
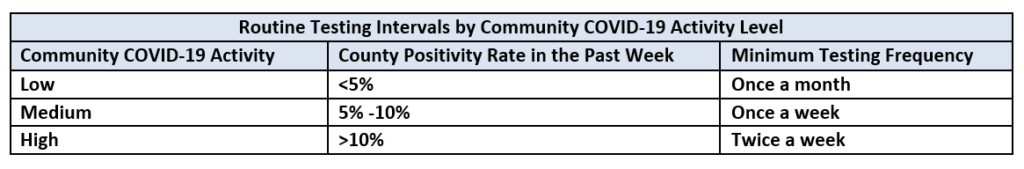
The frequency of testing once or twice a week presumes availability of POC testing onsite or when off-site testing turnaround time is less than 48 hours. If the 48 hour turnaround time cannot be met due to community testing supply shortages, limited access, or inability of laboratories to process tests within 48 hours, the facility should have documentation of its efforts to obtain quick turnaround test results with the identified laboratory or laboratories and the facility’s contact with the local and state health departments. Please contact Jan Kulik (jkulik@isdh.in.gov) at the Indiana State Department of Health regarding testing issues, and in the alternative Matt Foster ( mfoster@isdh.in.gov).
Importantly, nursing facilities should begin testing all staff the frequency prescribed in the Routine Testing table based on the county positivity rate when available starting August 28th, and facilities should monitor their county positivity rate every other week (i.e., first and third Monday of every month) and adjust the frequency of performing staff testing according to the following:
- If the county positivity rate increases to a higher level of activity, the facility should begin testing staff at the frequency shown in the table above as soon as the criteria for the higher activity is met.
- If the county positivity rate decreases to a lower level of activity, the facility should continue testing staff at the higher frequency level until the county positivity rate has remained at the lower activity level for at least two weeks before reducing testing frequency.
As a reminder, “staff” includes employees, consultants, contractors, volunteers, and students in the facility’s CNA training program or from affiliated academic institutions. Only individuals who work onsite at the facility will be subject to the testing requirements. Nursing facilities will be required to document each instance of staff or resident COVID-19 testing. If a vendor or volunteer is tested by another source (i.e., their own employer), the facility is required to obtain documentation that the testing was completed.
Generally, for all testing, staff and residents who previously tested positive for COVID-19 do not need to be retested for three (3) months after the date of symptom onset with the prior infection.
Refusal of Testing
Nursing facilities must have procedures in place to address staff who refuse testing. Procedures should ensure that staff who have signs or symptoms of COVID-19 and refuse testing are prohibited from entering the building until the Return to Work Criteria are met. If outbreak testing has been triggered and a staff member refuses testing, the staff member should be restricted from the building until the procedures for outbreak testing have been completed. The facility should follow its occupational health and local jurisdiction policies with respect to any asymptomatic staff who refuse routine testing. Residents (or their designated representative) may exercise their right to decline COVID-19 testing, but if they refuse must be placed in transmission based precautions until criteria for discontinuation of such precautions is met.
Reporting Test Results
Facilities conducting tests under a CLIA certificate of waiver are subject to regulations that require laboratories to report data for all testing completed, for each individual tested. For additional information on these reporting requirements, please click here. In addition to CLIA reporting requirements, nursing facilities should continue to report to NHSN on a weekly basis and to the ISDH within 24 hours of knowledge of a positive COVID-19 case or death confirmed with a PCR test.
Documentation of Testing
Facilities must demonstrate compliance with the testing requirements through the following documentation:
- For symptomatic residents and staff, document the date(s) and time(s) of the identification of signs or symptoms, when testing was conducted, when results were obtained, and the actions the facility took based on the results.
- Upon identification of a new COVID-19 case in the facility (i.e., outbreak), document the date the case was identified, the date that all other residents and staff are tested, the dates that staff and residents who tested negative are retested, and the results of all tests.
- For routine staff testing, document the facility’s county positivity rate, the corresponding testing frequency indicated, the date each positivity rate was collected, the date(s) that testing was performed for all staff, and the results of each test.
- Document the facility’s procedures for addressing residents and staff that refuse testing or are unable to be tested, and document any staff or residents who refused or were unable to be tested and how the facility addressed those cases.
- When necessary, such as in emergencies due to testing supply shortages, document that the facility contacted state and local health departments to assist in testing efforts, such as obtaining testing supplies or processing test results.
Updated Survey Tools
Going forward, surveyors will ask for a facility’s documentation as outlined above and will review a sample of staff and resident records. Surveyors are also encouraged to observe testing in real-time or at least interview an individual responsible for testing to inquire how testing is conducted. If the facility has a shortage of testing supplies, or cannot obtain test results within 48 hours, the surveyor will ask for documentation that the facility contacted state and local health departments to assist with these issues. Noncompliance related to these new testing requirements will be cited at new tag F886. If the facility has documentation that demonstrates their attempts to perform and/or obtain testing in accordance with these guidelines (i.e.., timely contacting state officials, multiple attempts to identify a laboratory that can provide testing results within 48 hours), surveyors should not cite the facility for noncompliance and should inform the state or local health department of the facility’s lack of resources.
IHCA/INCAL staff will continue to review this QSO and update this article as more information is learned and any additional guidance is issues.


